Drug therapies are now widely used to treat inner ear disorders. Other drugs that may potentially be useful in the ear are also being studied.
Therapies are typically separated according to whether the drug is given systemically or applied directly to the ear. Systemic applications include those given orally or intravenously. Local applications to the ear are commonly given by intratympanic delivery but also includes drugs delivered directly into perilymph, such as from cochlear implants. Drugs that have a target specifically in the ear and whose effects on other organ systems are negligible would normally be given systemically. More commonly, when the drug affects multiple organ systems, local applications are more appropriate.
PK for Systemically Applied Drugs
Drug pharmacokinetic (PK) studies are called for when:
- A systemically applied drug is intended to target the ear.
- A systemically applied drug is intended to target another part of the body but has side effects on the ear, such as causing damage (ototoxicity).
- Where combinations of drugs are being used, such as an ototoxic therapeutic in combination with a protective agent to prevent ototoxicity.
In ear case, perilymph samples are collected from the ear to determine the amounts of drug gaining access to the ear. Often the samples are collected at different times after drug administration to establish the concentration time course.
PK for Locally Applied Drugs
In general, delivering drugs locally to the ear allows smaller amounts of drug to be applied, minimizes systemic side-effects, and in many cases allows higher drug concentrations to be achieved in the ear. Each drug has different distribution characteristics when applied to the ear, depending on its molecular properties. Therefore, PK studies, measuring the concentration and distribution of each drug in the ear are necessary to ensure a therapeutic concentration of drug is achieved. A simplified example of the measurements and decisions is given in the flowchart below.
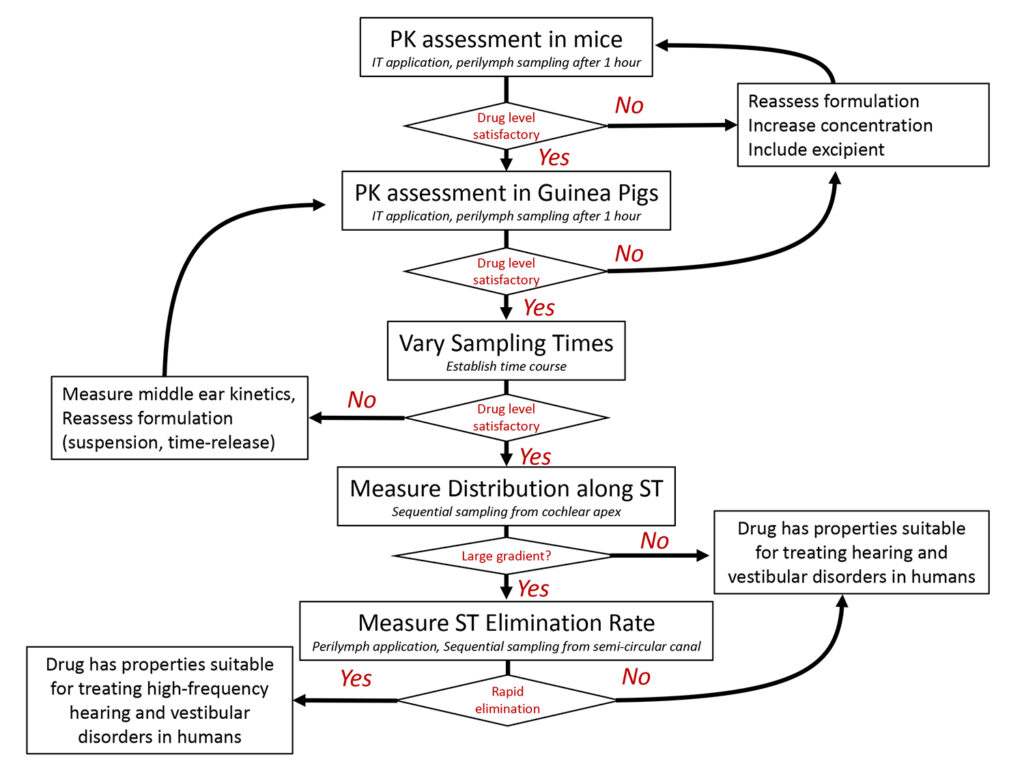
1) Initial Assessment
In initial studies, a formulation of the drug is applied IT, usually in mice. After a delay of up to 3 hours, perilymph of the ear is sampled and analyzed. These results give the first indication of whether a therapeutic concentration of the drug can be achieved in the ear.
If the drug level is insufficient, then the formulation and delivery protocol is reassessed. Drug concentration may be increased or an excipient (such as a tissue permeabilizing agent) may be added to increase drug penetration through the round window membrane. However, one limitation of experiments in mice is that inner ear volumes and applied drug volumes are both small, which can result in faster kinetics. Proceeding with measurements in guinea pigs, where larger volumes can be applied, can be considered.
In either case, more detailed PK studies are commonly performed in guinea pigs. In initial studies in guinea pigs the IT formulation is applied, and perilymph is usually sampled after a 1 hr or 3 hr delay.
If drug concentration falls within an acceptable range, then sampling perilymph after longer delay times (6 hr, 24 hr, 7 days, 14 days, 28 days, etc) can proceed. For drugs which bind to tissue components, it may also be necessary to measure tissue drug levels (by homogenizing the entire cochlea) to determine if they differ from the free drug level in perilymph. Lipophilic drugs, which bind to membrane lipids tend to accumulate in the tissues.
2) Separation of PK Components
If perilymph drug levels are low, or if drug is lost from perilymph more rapidly than is desired, then the underlying process causing the problem must be identified.
Measurements of middle ear contents with time will establish whether the drug is being lost rapidly from the middle ear, a relatively common occurrence. Solutions to middle ear loss can be to deliver drug as a suspension (which dissolves slowly over time) or in the form of a timed-release formulation (also providing a prolonged release duration)
If perilymph drug levels are low, substances such as benzyl alcohol or saponin can be included in the formulation to permeabilize the round window membrane and increase entry.
A rapid loss from perilymph is also possible, considered further below.
3) Longitudinal Gradient Measurement
In all of the above measurements, perilymph collection involves taking a single sample from each ear for analysis, maximizing the volume available for the assay. A more detailed analysis of drug distribution along the cochlea can be obtained by the procedure of “Sequential Sampling”, as shown below.
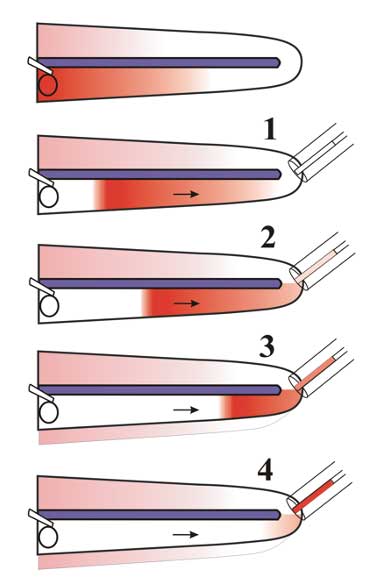
In this technique, the cochlear apex is perforated, and repeated small samples of perilymph are collected. Each sample takes about 60 sec to accumulate, and each is analyzed separately. The perilymph is being pushed out by cerebrospinal fluid, entering scala tympani at the base of the cochlea. The samples therefore represent perilymph, initially from the apical regions and then from progressively more basal regions as shown in the figure. Analysis of the samples allows drug gradients along the ear to be quantified.
Drug gradients make it more difficult to treat apical cochlear regions. Furthermore, if drug gradients exist along the length of the guinea pig ear, then the gradients along the human ear are likely to be twice as large, as the human cochlea is approximately twice the length of the guinea pig.
4) Perilymph Elimination Measurement
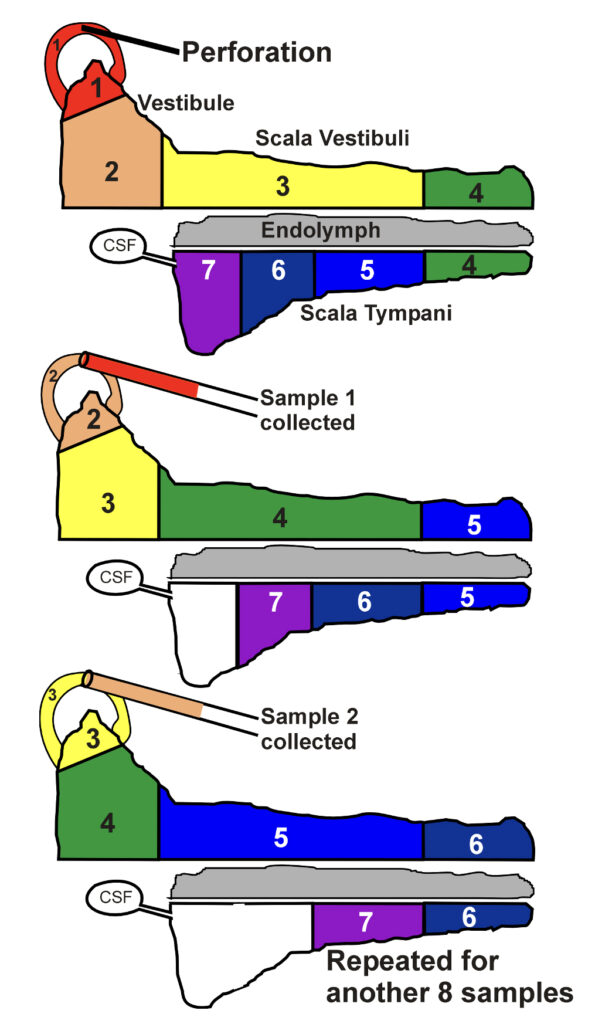
Although the rate of elimination of drug from the perilymph space is the primary cause of drug gradients along the ear, measurement of elimination rate is remarkably difficult. It cannot be quantified in experiments with IT delivery as the gradients also depend on other factors, such as the rate of entry at the RW and changes in middle ear concentration. We have found that the only reliable method to quantify elimination rate is to load the entire perilymphatic space and adjacent tissue spaces with drug. This is performed by injecting drug solution into a semi-circular canal. The decline of perilymph concentration with time gives a good estimate of elimination rate. Perilymph is again collected by sequential sampling, in this case from the canal site where drug was injected, as shown schematically below. Elimination rates for different regions of the inner ear can be derived from experiments in which perilymph is sampled at different times after loading.
The PK measurements of elimination rates and how the drug distributes in the ear can be interpreted quantitatively with the FluidSim computer model. When appropriate parameters have been established, FluidSim can then make predictions for how the drug is likely to distribute in the human ear.
In general, drugs that are retained in perilymph well are more suitable for treating auditory disorders relative to “leaky” drugs that are lost more readily. Leaky drugs do not distribute very far apically along the ear. They are not good candidates for treating auditory disorders affecting the lower frequency regions important to understanding speech.