Explant (ex vivo) cultures allow for cost-effective, high-throughput screening of drugs for the ears.
The most relevant approach to evaluating treatments for the ears is to test these drugs and devices using intact auditory systems. However, before such in vivo studies it is common to first conduct cochlear explant studies because they can provide an excellent method to rapidly and effectively screen a large number of test article formulations and concentrations.
An otic explant study often begins by harvesting cochlear tissue from early post-natal mice, and placing each cochlea in culture conditions that include a combination of test article(s), negative control compounds, and/or positive control compounds. After several days, samples are fixed, immunostained, and analyzed microscopically to quantify hair cells, auditory nerve fibuers, analyze cellular health and nuclear condensation, or perform other analyses.
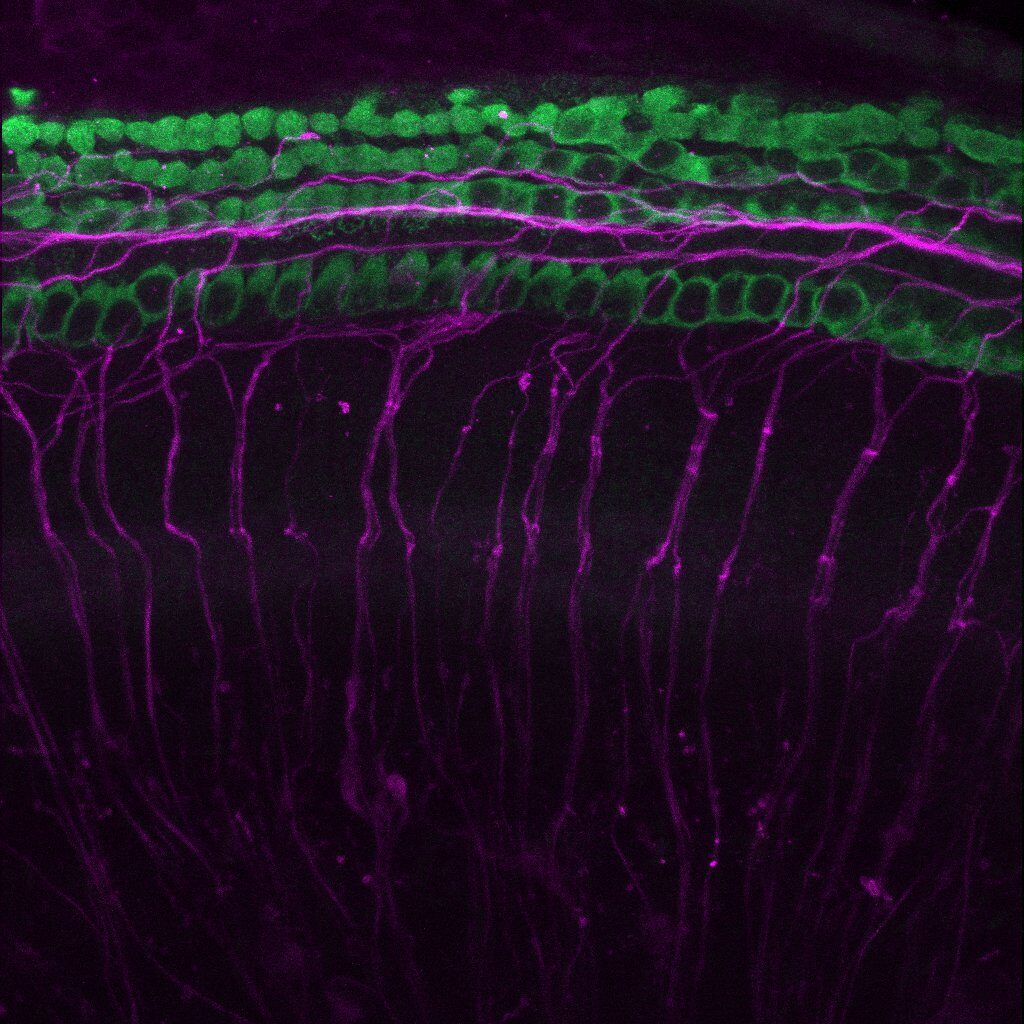
The objective of each protocol is to create a culture environment that closely models actual in vivo conditions. Unlike using cell line cultures, explant samples maintain much of the natural tissue architecture, and allow cells to produce their normal cytokines and growth factors as much as possible. The benefits of explant studies are significant – a sponsor can rapidly obtain information about a test article’s IC50/EC50, toxicity potential, and ability to protect fragile cochlear cells in a cost-effective manner.

